|
Replies
|
wrote...
|
|
|
6 years ago
|
The moment I read your topic title, I thought of the chemical benzene, then I thought of cholesterol molecules, then I thought of the nitrogenous bases found in DNA and RNA because all three noted examples also contain molecules that are hexagonal shapes. So maybe we can explain this phenomenon at the molecular level rather than at the biological level, such as the honeycombs discussed in the video. There is a strong mathematical correlation to the hexagon shapes we see in nature and stability attributed to the shape.
In the case of carbon, the only molecule that adopts a perfect hexagonal geometry in its ground state is benzene. In this case the hexagonal geometry is adopted because all of the carbons are sp2 hybridized.
S = spherical orbitals
P = peanut-shaped orbitals
In any shell (1,2,3, etc) there is 1 S orbital and 3 P orbitals. Each P orbital exists in either the X, Y, or Z direction. sp2 means that you mixed an S orbital with 2 p's, so your peanut isn't so lopsided
Anyway, the ideal geometry (lowest energy) for an sp2 hybridized carbon involves three 120 degree bond angles around the carbon atom. This can be accomplished in the case of benzene by placing 6 carbon atoms in a plane and connecting them as shown to produce the benzene structure.
That's the reason why octagons are rarely found in molecular chemistry. These atoms prefer to exist in a geometry with 3 equivalent 120 degree bond angles. The internal angle in a regular pentagon and a regular octagon are 108 and 135 degrees respectively. Since these angles deviate from the 120 degree ideal, these molecules will be strained, unlike benzene.
|
|
|
|
|
|
|
- Master of Science in Biology
- Bachelor of Science
|
|
|
wrote...
|
|
|
6 years ago
|
Hi guys
I think the video summarizes it pretty well, at the very end he says that nature seeks out the shape with the lowest energy. That makes sense biologically: invest the least amount of energy for the optimal outcome. Also, bees don't make honeycombs, as mentioned, except they form circles that position themselves into combs due to heat.
|
|
|
|
|
|
|
|
|
wrote...
|
|
|
6 years ago
I would like to know on how shape is formed based on the pressure and tension force, please see following image for details.  Does anyone have any suggestions? Thanks, to everyone very much for any suggestions (^v^)
|
|
|
|
|
|
|
wrote...
|
|
|
6 years ago
|
probably coincidental because you see many symmetrical shapes in nature both at the molecular and structural level
for example, most animal viruses have 'icosahedral' capsids: they are polyhedrons with 20 identical equilateral triangular faces
The protozoa Radiolarian also has this icosahedral shape. So there are many symmetrical objects in nature compared to curved shapes.
If we venture into the structures of elements, elemental boron has icosahedral symmetry. The crystalline shapes of naturally occurring elements, especially the transition metals are symmetrical.
Transition metal complexes (coordination complexes) for example, [Cu(H2O)6]2+ or any number of transition metals that you may have seen represented as 'hydrates' (CuCl2.6H2O for example) exist in water as octahedrons.
Then there are square planar molecules like gold complexes or platinum complexes which exist in nature and are quite square in their molecular geometry and we can see these exist in nature by X-ray crystallography.
Even proteins, while they may be folded, have portions that are rectangular (parts of their secondary structure called 'beta-sheets' comprised of beta-strands).
hope this helps
|
|
|
|
|
|
|
wrote...
|
|
|
6 years ago
I would like to know on how shape is formed based on the pressure and tension force, please see following image for details. A few things just to clarify, when you mention shape (quoted above), are you still referring to hexagons, as in the video where he blew water bubbles and they formed 120 degree angles (like the interior angles of a hexagon)? If so, bubbles and soap films are made of water (with a skin of soap molecules) and surface tension pulls at the liquid surface to give it as small an area as possible. That’s why raindrops are spherical (more or less) as they fall: A sphere has less surface area than any other shape with the same volume. On a waxy leaf, droplets of water retract into little beads for the same reason. This surface tension explains the patterns of bubble rafts and foams. The foam will seek to find the structure that has the lowest total surface tension, which means the least area of soap-film wall. But the configuration of bubble walls also has to be mechanically stable: The tugs in different directions at a junction have to balance perfectly. The pressure of the gas inside a cell or bubble gets bigger as the bubble gets smaller, so the wall of a small bubble next to a larger one will bulge outward slightly. Furthermore, I only seem to understand the two pictures on the right side, where a single molecule of water is more content when equally surrounded by like molecules 
|
|
|
|
|
|
|
|
|
wrote...
|
|
|
6 years ago
Referring to following linked image, video has mentioned to compare different geometry for minimizing the perimeter relative to number of cells 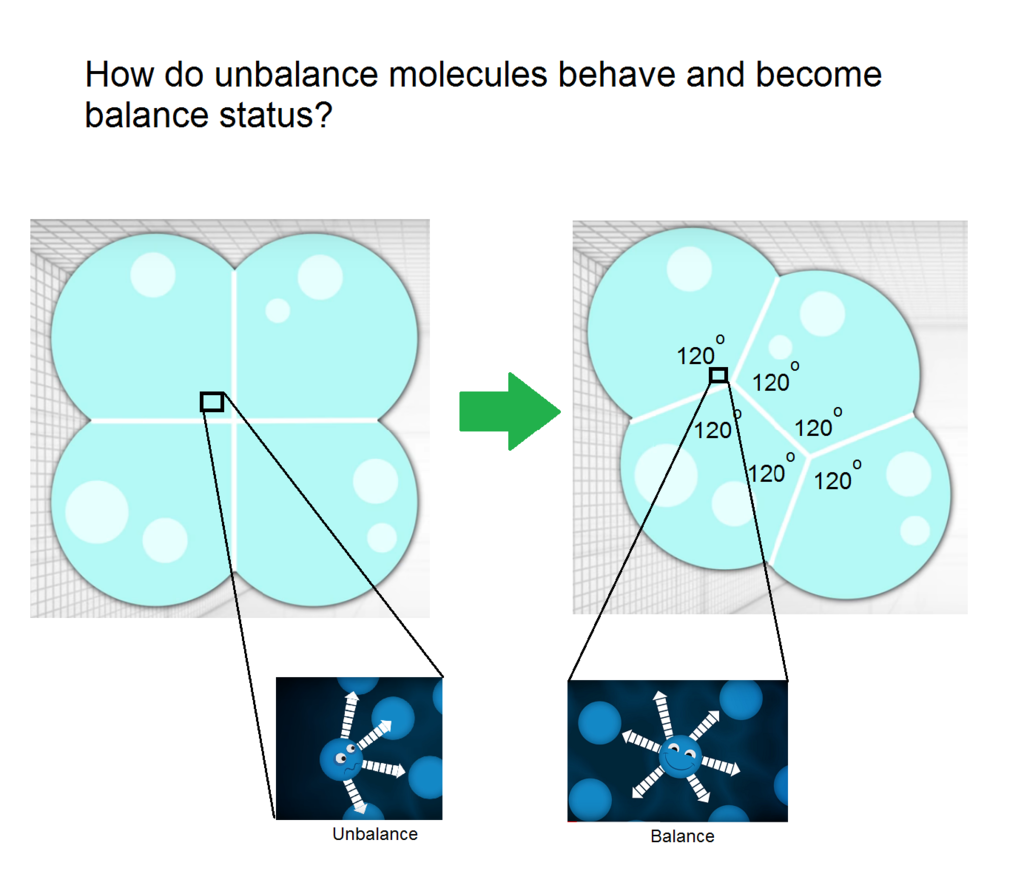 Do you have any suggestions on what happen occur between tension and force? Thanks, to everyone very much for any suggestions (^v^)
|
|
|
|
|
|
|
wrote...
|
|
|
6 years ago
|
The image link is corrupt, please consider uploading it instead.
In biology, cells are designed to be tiny (take on the least perimeter) so they can maximize their ratio of surface area to volume. Smaller cells have a higher ratio which allow more molecules and ions move across the cell membrane per unit of cytoplasmic volume. Generally speaking, cells are so small because they need to be able to get the nutrients in and the waste out quickly.
Now in terms of tension and force, I don't see a correlation. Where did you get this idea of tension and force, I'm confused.
|
|
|
|
|
|
|
|
|
wrote...
|
|
|
6 years ago
Referring to following linked image, video has mentioned to compare different geometry for minimizing the perimeter relative to number of cells  Do you have any suggestions on what happen occur between tension and force? Thanks, to everyone very much for any suggestions (^v^)
|
|
|
|
|
|
|
wrote...
|
|
|
6 years ago
Edited: 6 years ago, bio_man
|
What makes you think the molecules within a bubble are uniform?
Soap bubble physics is a complicated matter involving the Double bubble conjecture, Plateau's laws, Young–Laplace equation, mean curvature, etc.
When two bubbles merge, they adopt a shape which makes the sum of their surface areas as small as possible, compatible with the volume of air each bubble encloses. If the bubbles are of equal size, their common wall is flat. If they aren't the same size, their common wall bulges into the larger bubble, since the smaller one has a higher internal pressure than the larger one, as predicted by the Young–Laplace equation.
At a point where three or more bubbles meet, they sort themselves out so that only three bubble walls meet along a line. Since the surface tension is the same in each of the three surfaces, the three angles between them must be equal to 120°. All these rules, known as Plateau's laws, determine how a foam is built from bubbles.
That being said, if bubbles makes 120 degrees coming together, we can assume that the amount of molecules into the three are equal. How they balance themselves out is completely random because in a soap bubble, there's air, and air consists mostly of O2, an inert gas.
|
|
|
|
|
|
|
|
|
|
wrote...
|
|
|
6 years ago
Referring to following linked image, video has mentioned to compare different geometry for minimizing the perimeter relative to number of cells Your question here jumps from one thing to another. It starts with bubbles, then you mention cells. I'm confused. In the illustration you provide, you show perfect spherical soap bubbles. Is that part of the criteria? In other words, are the radii the same? You'll need to clarify before I can offer more details.
|
|
|
|
|
|
- Master of Science in Biology
- Bachelor of Science
|
|
|