It seems that color / wavelength are fixed based on absorb and radiate energy levels. so why apple is red? which orbit levels are related to absorb and radiate energy (red color wavelength)? is the distance between orbit levels fixed within N2 (Blue) and O2 (Yellow or Orange) structure?
What makes an apple red or green depends on the molecules contained in the pigment making up the apple. I'm not sure what makes up the red color in apples. Nitrogen and oxygen aren't the only elements that produce a certain color.
which orbit levels are related to absorb and radiate energy (red color wavelength)? is the distance between orbit levels fixed within N2 (Blue) and O2 (Yellow or Orange) structure?
My knowledge in quantum physics is quite limited.
Furthermore, when red color wavelength do not change any orbit levels within N2 or O2 atom, does red color wavelength just pass through atom without any interaction with atom's electrons?
Probably it reflects off the atom's nucleus while the other wavelengths are absorbed.
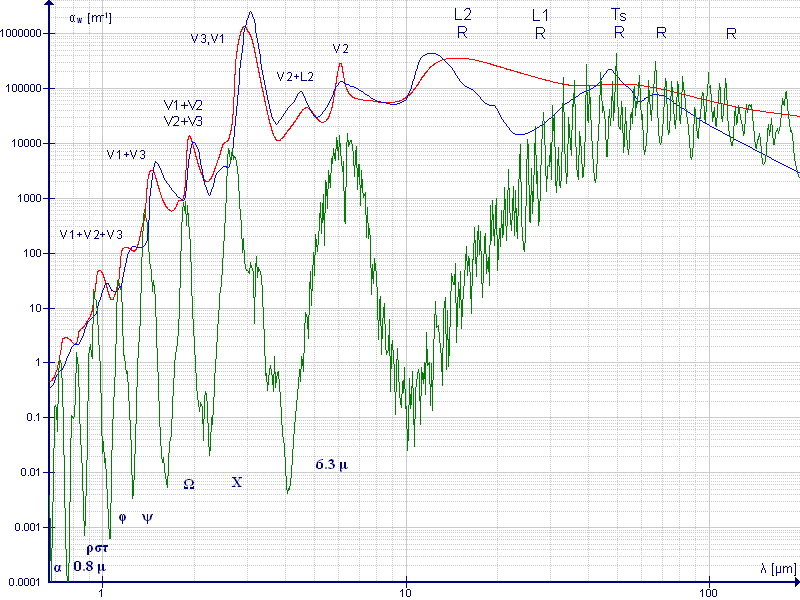
I would like to check H2O absorption spectrum and confirm on whether blue color from ocean is related to H2O absorption spectrum or not.
When you know on how to use the reference, could you please also check on H2O absorption spectrum as well?
Source:
LinkCaption:
Absorption spectrum (attenuation coefficient vs. wavelength) of liquid water (red), atmospheric water vapor (green) and ice (blue line) between 667 nm and 200 μm.