Since light can travel through vacuum, is Light a particle, not wave, would it be correct? but light is vibrated at high frequency, which can knocks off an electron within atom. If light is defined as electromagnetic radiation, light have both property of particle and wave.
Ocean is blue
Light contains different color of spectrum

Electron Movement
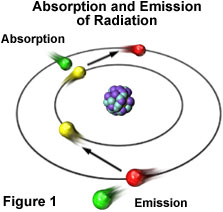
We can only see object from reflected energy, so somehow ocean reflects a lot of blue color, so we see blue ocean. On the other words, atom emits blue light wavelength frequency and most of electrons emit blue light by dropping into lower energy levels.
I would like to know on why (ocean is not red) or (H2O has less red light wavelength emission), does ocean (H2O) has less electron at lower energy levels?
Furthermore, back to the basic question, how do Rayleigh scattering work on atom level?
Do you have any suggestions?
Thanks, to everyone very much for any suggestions :>
Referring to following video, when an free electron knocks off Hg's electron, that Hg's electron would move up one energy level. After the free electron gives a ultraviolet proton to Hg's electron (as video mentioned). I would like to know on what the free electron get or lose during this process. A free electron is just an electron, why a free electron can give out a ultraviolet proton on above process?
Do you have any suggestions?
Thanks, to everyone very much for any suggestions :>