Definition for Porphyrin
From Biology Forums Dictionary
A porphyrin is an organic compound that contains four pyrrole rings. A pyrrole is a pentagon-shaped ring of four carbon atoms with a nitrogen atom at one corner (C4H5N).
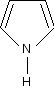
Pyrrole.
The porphyrins are actually an enormous group of organic compounds, found all over the living world. The special thing about porphyrins is that they bind metals. The four nitrogens in the middle of the porphyrin molecule act as teeth: they can grab and hold metal ions such as magnesium (Mg), iron (Fe), zinc (Zn), nickel (Ni), cobalt (Co), copper (Cu), and silver (Ag).
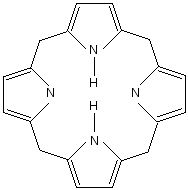
Porphyrin is a compound made of four pyrrole rings.
When a porphyrin molecule grabs a metal, it acquires different properties and gets a different name. So, if the central metal is iron (Fe), the porphyrin complex is called ferroporphyrin, or heme. Hemoglobin, the molecule in your red blood cells that grabs oxygen, has four of these heme groups. The iron ion of the heme group is responsible for binding the oxygen. The hemoglobin travels around the body, and when it reaches the tissues the iron in the porphyrin complex releases the oxygen to nourish them.
Chlorophyll, the all-important molecule that allows plants to capture the sun's energy, and is responsible for plants' green color, is made up in part of a porphyrin molecule whose central metal ion is magnesium (Mg). The vitamin B12 consists of a porphyrin molecule with a cobalt ion in the middle.
So, porphyrins are an extremely important group of organic compounds. They are universal, found in most living cells of animals and plants, where they perform a wide variety of functions.
