Definition for Disulfide bond
From Biology Forums Dictionary
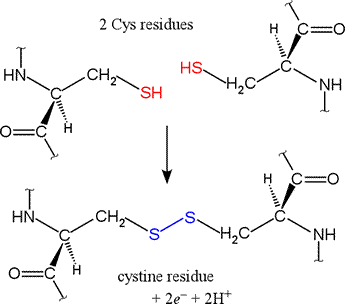
A disufide bond is a covalent chemical bond between two sulfur atoms derived from two sulfhydryl or thiol groups.
In biochemistry, these thiol groups are usually from the side chains of the amino acid cysteine. Shown at right are two cysteine residues in polypeptide chain(s). The thiol groups are in their reduced forms (in red in figure). Removal of two hydrogens (H+ + e−) from each thiol (by an oxidizing agent, not included in the figure, which represents an oxidation half-reaction), and concomitant formation of a new covalent bond - the disulfide bond - between the two sulfur atoms yields the lower structure at right. A disulfide-linked pair of cysteine residues is termed a cystine residue. The conversion of two sulfhydryl groups to a disulfide linkage is an oxidation reaction. Conversely, disulfide bonds can be reduced to yield two thiols, which is the reverse of the half-reaction shown at right.