Definition for Nicotinamide adenine dinucleotide
From Biology Forums Dictionary
» Redirected from NAD
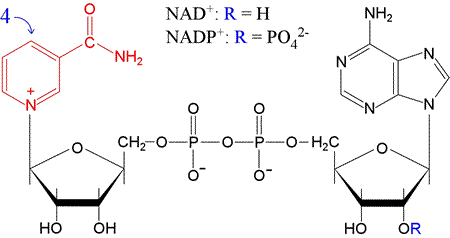
An important, ubiquitous redox cofactor that functions as a carrier of electron pairs. The oxidized form of the cofactor actually carries a positive charge, and is denoted NAD+ while the reduced form is NADH. The nicotinamide portion of NAD+, which consists of a carbamylated pyridine ring (in red in the figure below), acts as the electron pair acceptor. The 4-position of the ring (para to the nitrogen atom) in effect becomes substituted with a hydride ion. Note that the "dinucleotide" part of the name is due to the fact that the other "half" of NAD is an adenine-containing nucleotide, AMP. The two "halves" of the molecule are linked by a phosphoanhydride bond.
A closely related cofactor, NADP+, (reduced form, NADPH) differs only in having a phosphate attached to the 2′ position of the ribose attached to adenine. NADPH generally functions in redox reactions in biosynthetic pathways (e.g. fatty acid synthesis) , whereas NAD+ predominates in catabolic processes, such as those associated with glycolysis and oxidative phosphorylation.
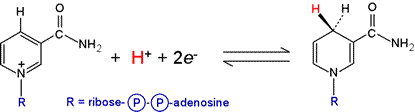
The NAD+/NADH redox half-reaction (see figure below) has a standard biochemical reduction potential, ΔE°′ of −0.315 V. The progress of reactions involving NAD+/NADH can be conveniently monitored spectrophometrically due to the appearance of a broad absorption with its peak at 340 nm when NADH is formed.
The major source of NADH in oxidative metabolism is the citric acid cycle. The NADH produced is reoxidized to NAD+ when the former donates its electrons to the first component of the electron transport chain. Eventually these electrons reduce molecular oxygen to water.
