Definition for Starling's curve
From Biology Forums Dictionary
As described elsewhere, Cardiac output increases or decreases in response to changes in heart rate or stroke volume. When a person stands up, for example, cardiac output falls because of a fall in central venous pressure, which leads to a decrease in stroke volume. As another example, limb movement (muscle pump) during exercise enhances venous return to the heart, which causes an increase in stroke volume. What is the mechanism by which changes in venous return alters stroke volume?
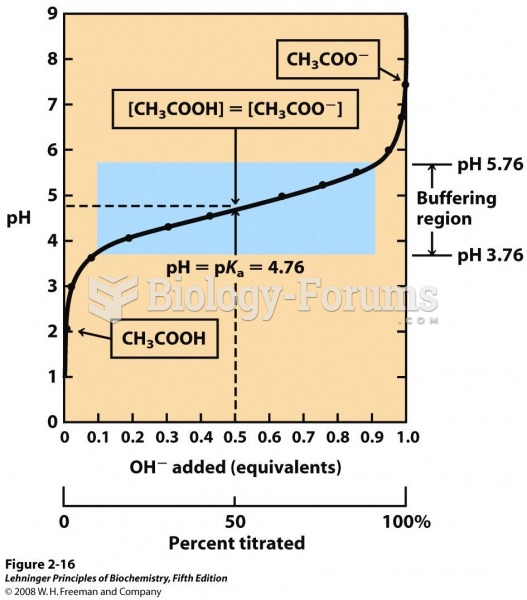
In the late 19th century, Otto Frank found using isolated frog hearts that the strength of ventricular contraction was increased when the ventricle was stretched prior to contraction. This observation was extended by the elegant studies of Ernest Starling and colleagues in the early 20th century who found that increasing venous return, and therefore the filling pressure of the ventricle, led to increased stroke volume in dogs. These cardiac responses, which occur in isolated hearts as well as in intact animals and humans, are independent of neural and humoral influences on the heart. In honor of these two early pioneers, the ability of the heart to change its force of contraction and therefore stroke volume in response to changes in venous return is called the Frank-Starling mechanism (or Starling's Law of the heart).
Increased venous return increases the ventricular filling (end-diastolic volume) and therefore preload, which is the initial stretching of the cardiac myocytes prior to contraction. Myocyte stretching increases the sarcomere length, which causes an increase in force generation. This mechanism enables the heart to eject the additional venous return, thereby increasing stroke volume.
This phenomenon is described in mechanical terms by the length-tension and force-velocity relationships for cardiac muscle. Increasing preload increases the active tension developed by the muscle fiber and increases the velocity of fiber shortening at a given afterload and inotropic state.
One mechanism to explain how preload influences contractile force is that increasing the sarcomere length increases troponin C calcium sensitivity, which increases the rate of cross-bridge attachment and detachment, and the amount of tension developed by the muscle fiber (see Excitation-Contraction Coupling). The effect of increased sarcomere length on the contractile proteins is termed length-dependent activation.
It has traditionally been taught that the Frank-Starling mechanism is due to changes in the number of overlapping actin and myosin units within the sarcomere as in skeletal muscle. According to this view, changes in the force of contraction do not result from a change in inotropy. Because we now know that changes in preload are associated with altered calcium handling and troponin C affinity for calcium, a sharp distinction cannot be made mechanistically between length-dependent (Frank-Starling mechanism) and length-independent changes (inotropic mechanisms) in contractile function.

There is no single Frank-Starling curve on which the ventricle operates. There is actually a family of curves, each of which is defined by the afterload and inotropic state of the heart. For example, increasing afterload or decreasing inotropy shifts the curve down and to the right. Decreasing afterload and increasing inotropy shifts the curve up and to the left. To summarize, changes in venous return cause the ventricle to move along a single Frank-Starling curve that is defined by the existing conditions of afterload and Inotropy.

Frank-Starling curves show how changes in ventricular preload lead to changes in stroke volume. This graphical representation, however, does not show how changes in venous return affect end-diastolic and end-systolic volume. In order to do this, it is necessary to describe ventricular function in terms of pressure-volume diagrams. When venous return is increased, there is increased filling of the ventricle along its passive pressure curve leading to an increase in end-diastolic volume. If the ventricle now contracts at this increased preload, and the afterload is held constant, the ventricle will empty to the same end-systolic volume, thereby increasing its stroke volume. The increased stroke volume is manifested by an increase in the width of the pressure-volume loop. The normal ventricle, therefore, is capable of increasing its stroke volume to match physiological increases in venous return. This is not, however, the case for ventricles that are in failure.