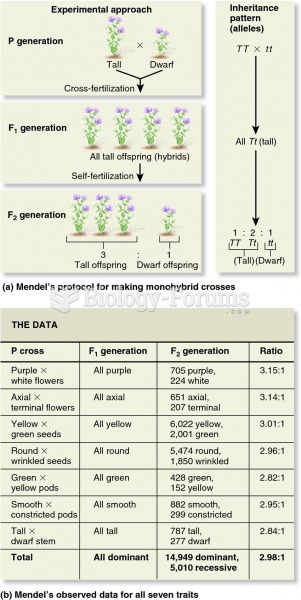
Definition for Dilution factor
From Biology Forums Dictionary
Ratio of final volume/aliquot volume (final volume = aliquot + Diluent).
To calculate a dilution factor:
Remember that the dilution factor is the final volume/aliquot volume.
EXAMPLE: What is the dilution factor if you add 0.1 mL aliquot of a specimen to 9.9 mL of diluent?
- The final volume is equal the the aliquot volume plus the diluent volume: 0.1 mL + 9.9 mL = 10 mL
- The dilution factor is equal to the final volume divided by the aliquot volume: 10 mL/0.1 mL = 1:100 dilution (102)
The Concentration Factor for this problem = aliquot volume/final volume = 0.1/(0.1 + 9.9) = 0.01 or 10-2 concentration