The trp operon
of E. coli controls
the biosynthesis of tryptophan in the cell from the initial precursor chorismic
acid. This operon contains genes for the production of five proteins which are
used to produce three enzymes. The products of the E and D genes
form a multimeric protein comprised of two copies of each protein to produce the
enzyme anthranilate synthetase. This enzyme catalyzes the first two reactions in
the tryptophan pathway. The next enzyme, which is responsible for catalyzing the
next two steps in the pathway is indole glycerolphosphate synthetase and it is
the product of the C locus.
The final step in the reaction is the pathway produces tryptophan from
indole-glycerol phosphate and serine. This single step is catalyzed by
tryptophan synthetase, an enzyme that is a multimer of two proteins that are the
product of the B and A genes.
As with all operons, the trp operon
consists of the repressor, promoter, operator and the structural genes. In this
system, though, unlike the lac operon,
the gene for the repressor is not adjacent to the promoter, but rather is
located in another part of the E.
coli genome. Another difference
is that the operator resides entirely within the promoter
Control Circuit for the trp Operon
P/O | L || E | D | C | B | A |
_____________________________________________________
Controlling Region || Structural genes
| trp Operon
Gene |
Gene Function |
|
P/O
|
Promoter; operator
sequence is found in the promoter |
|
trp L
|
Leader sequence;
attenuator (A) sequence is found in the leader |
|
trp E
|
Gene for anthranilate
synthetase subunit |
|
trp D
|
Gene for anthranilate
synthetase subunit |
|
trp C
|
Gene for
glycerolphosphate synthetase |
|
trp B
|
Gene for tryptophan
synthetase subunit |
|
trp A
|
Gene for tryptophan
synthetase subunit |
The trp operon
is a repressible system. The
primary difference between repressible and inducible systems is the result that
occurs when the effector molecule binds to the repressor. With inducible
systems, the binding of the effector molecule to the repressor greatly reduces
the affinity of the repressor for the operator, the repressor is released and
transcription proceeds. The lac operon
is an example of an inducible system. With repressible systems, the binding of
the effector molecule to the repressor greatly increases the affinity of
repressor for the operator and the repressor binds and stops transcription.
Thus, for the trp operon
, the addition of tryptophan (the effector molecule) to the E.
coli environment shuts off the
system because the repressors binds at the operator.
Inducible system - the
effector molecule interacts with the repressor protein such that it can not bind
to the operator
Repressible system - the
effector molecule interacts with the repressor protein such that it can bind to
the operator
Attenuation of the trp Operon
One element of the trp operon
is the leader sequence (L) that in immediately 5' of the trpE gene.
This sequence about 160 bp is size also controls the expression of the operon
through a process called attentuation.
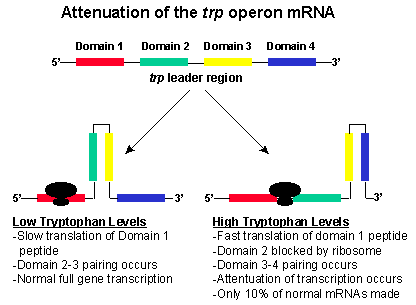
This sequence has four domains (1-4). Domain 3 (nucleotides 108-121) of the mRNA
can base pair with either domain 2 (nucleotides 74-94) or domain 4 (nucleotides
126-134). If domain 3 pairs with domain 4, a stem and loop structure forms on
the mRNA and transcription stops. This structure forms when the level of
tryptophan is high in the cell. If domain 3 pairs with domain 2, then the stem
and loop structure does not form and transcription continues through the operon,
and all of the enzymes required for tryptophan biosynthesis are produce. These
events occur when tryptophan is low in the cell.
If domain 4 is deleted, the stem and loop structure can not form and
transcription of the remainder of the operon will occur even in the presence of
tryptophan. Domain 4 is called the attenuator because
its presence is required to reduce (attenuate) mRNA transcription in the
presence of high levels of tryptophan.
Domain 1 is also an important component of the attenuation process. The
section of the leader sequence encodes a 14 amino acid peptide that has two
tryptophan residues.
How does this entire attentuation process work? We will discuss the molecular
events that occur under conditions of high and low tryptophan.
trp Operon Transcription
Under High Levels of Tryptophan
When the cellular levels of tryptophan are high, the levels of the tryptophan
tRNA are also high. Immediately after transcription, the mRNA moves quickly
through the ribosome complex and the small peptide is translated. Translation is
quick because of the high levels of tryptophan tRNA. Because of the quick
translation, domain 2 becomes associated with the ribosome complex. Then domain
3 binds with domain 4, and transcription is attenuated because of the stem and
loop formation.
trp Operon Transcription
Under Low Levels of Tryptophan
Under low cellular levels of tryptophan, the translation of the short peptide
on domain 1 is slow. Because of the slow translation, domain 2 does not become
associated with the ribosome. Rather domain 2 associates with domain 3. This
structure permits the continued transcription of the operon. Then the trpE-A genes
are translated, and the biosynthesis of tryptophan occurs.