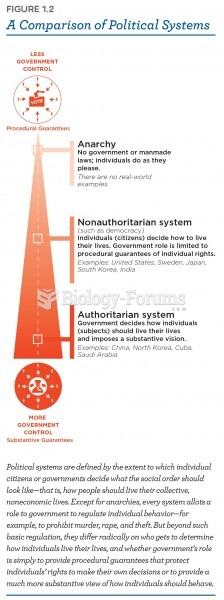
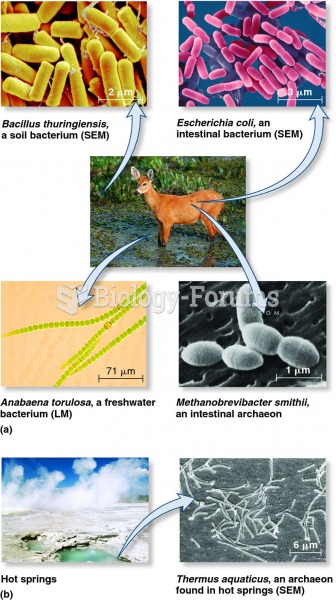
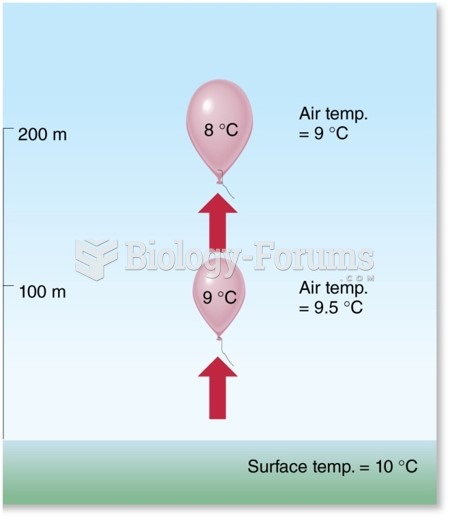
Definition for Comparison of Ionic and Covalent Compounds
From Biology Forums Dictionary
There are a number of differences in the physical properties of chemical compounds which depend on the type of bonds which are present in the molecules of the compound. These differences are summarised in the table below.
Ionic Compounds
- Formed by transferring electrons
- High melting and boiling points
- Form lattices
- Dissolve in water
- Do not dissolve in organic solvents
- Conduct electricity when melted or dissolved in water
- Strong forces holding whole ionic structure together
Covalent Compounds
- Formed by sharing electrons
- Low melting and boiling points
- No lattices, except diamond and graphite
- Do not dissolve in water
- Dissolve in organic solvents
- Do not conduct electricity (graphite is the only exception)
- Atoms in molecule held by strong forces, but weak forces between the molecules.