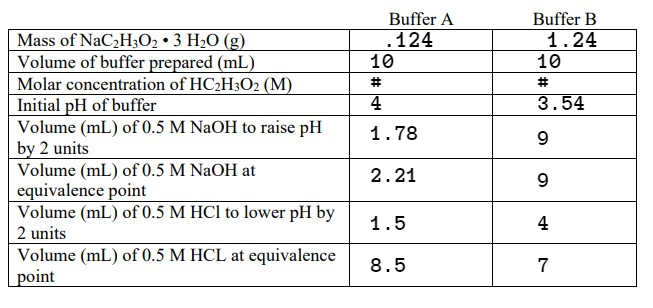

1. Find the
Molar concentration of HC2H3O2 (M) -I'm not sure if each buffers affect the molar concentration, but I searched up the normal molar concentration for HC2H3O2 and found that it is 0.8527 M. Can anyone confirm that or steer me in the right direction on how to solve for the molar concentration?
2. Using Excel, prepare two charts, each with two series, from your data.
Create a separate chart for your titrations using NaOH with a series plotting your
titration with Buffer A and a series plotting your titration with Buffer B.
Do the same with your titrations using HCl.
-I think I understand how to make the graphs: there will be 4 separate graphs, Buffer A - NaOH, Buffer A - HCl, Buffer B - NaOH & Buffer B - HCl. Please give me any advice if my thought process is on the right track.
3.
Write reaction equations to explain how your acetic acid-acetate buffer reacts with an acid and reacts with a base.4.
Buffer capacity has a rather loose definition, yet it is an important property of buffers. A commonly seen definition of buffer capacity is: “The amount of H+ or OH– that can be neutralized before the pH changes to a significant degree.”
We will consider 2 pH units to be significant. Use your data and your charts to determine the buffer capacity of Buffer A and Buffer B.-I don't expect anyone to do the work for me (especially for the graphs), just please give me advice on where to start, any formulas that may help, etc. 