Life is essentially a series of complex chemical reactions and molecular structures within cells. If life began
without a creator, for example, then it had to have formed from the chemicals present in the early earth whose atmosphere consists of mostly of nitrogen (N
2) and carbon dioxide (CO
2), but almost devoid of breathable oxygen (O
2). Coupled with this, the temperature was probably a lot hotter than the average of today.
It turns out that the organic molecules we are made from, including carbohydrates, lipids, proteins, and nucleic acids showcase a connection between these early molecules and the evolution of complex life forms. The continuity in the use of carbohydrates, lipids, proteins, and nucleic acids across different life forms suggests a common ancestry and the conservation of fundamental molecular processes.
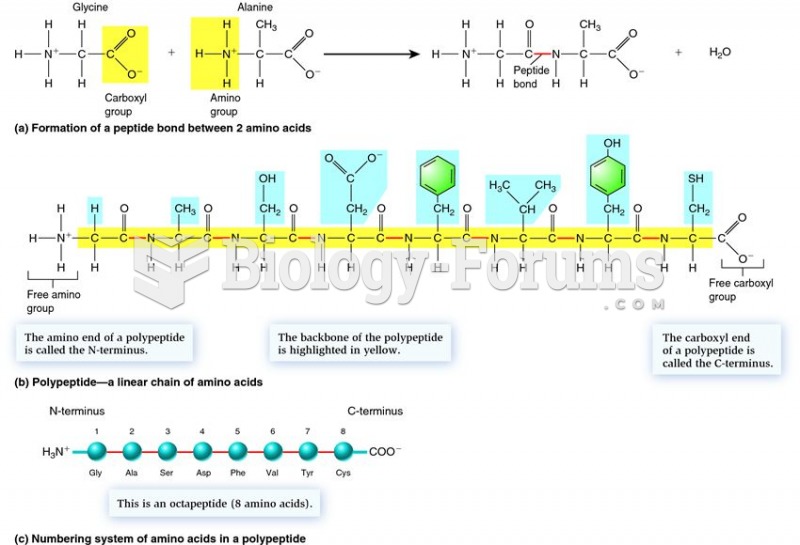
Carbohydrates, such as glucose, provide primary energy for cellular activities through processes like cellular respiration. In order to get energy from carbohydrates, like glucose, enzymes are required to break and harvest the energy from the bonds of these molecules. Similarly, lipids, including fats and phospholipids, contribute to cell membrane structure and long-term energy storage. Again, based on the chemical structure of these molecules, we have evolved proteins and enzymes that can break these elaborate molecules down and harvest their energy for life processes. Additionally, proteins, which are composed of amino acids, play key roles in catalyzing biochemical reactions, providing structural support, and acting as transport molecules. Proteins are made out of polypeptides which are chemically connected by peptide bonds. These peptide bonds are based on the same two elements making up N2 and CO2, namely carbon and nitrogen. Similarly, nucleic acids, like DNA and RNA, carry hereditary information, emphasizing the influence of chemical structure on the blueprint of life.
Enzymes are specialized proteins that act as catalysts in biological reactions. They do this by contorting biochemicals in such a way that speed up the reaction (i.e. via hydrolysis, condensation, forming bonds, etc.). pH, which is chemical a chemical measure of solution acidity or alkalinity, is crucial for maintaining the proper environment for biochemical reactions, impacting homeostasis – the ability of living systems to maintain a stable internal environment.
Does that help?